Progression-free survival (PFS) is a surrogate endpoint is defined as follows (by ChatGPT):
In essence, PFS refers to the length of time during a clinical study or treatment regimen where a patient’s disease doesn’t worsen or progress. For instance, in cancer research, it measures the time from the start of treatment until the cancer shows signs of growth, spread, or relapse.
Understanding PFS is vital because it helps clinicians and researchers assess the effectiveness of a treatment in controlling the disease. A longer PFS suggests that the treatment is successfully slowing down the progression of the illness. This metric is particularly important in clinical trials when comparing different treatment approaches or drugs.
A key question is, do patients care about PFS? The answer may be yes, because better PFS is most often (but not always) correlated with longer survival. However, do patients value PFS independent of OS? Perhaps so if their quality of life is better (i.e., they have fewer symptoms) during the pre-progression phase. Or, perhaps they have lower anxiety if they know their cancer has not progressed.
What does the literature say?
A paper by Raphael et al. (2019) conducted a systematic literature review of studies that evaluate whether patients with advanced cancer understand and value PFS. Overall, 17 studies met their inclusion criteria. Of these:
Ten studies specifically presented patients with the term progression-free survival as an attribute choice. In the words used to define the attribute of PFS, 6 studies used the term survival. Five studies clarified that PFS may not translate into better overall survival, and 5 studies explained that improvements in PFS may not reflect how well the patient may feel. No study clarified that a PFS event could represent either progression or death, and no study defined for the patient what constituted progression. The studies assessed herein underrepresented ethnic and racial minorities (mean percentage of white patients, 88%; range, 77%-96%). Values and preferences may vary across cultural backgrounds given that different relative preferences were assigned to cost and efficacy outcomes in North American vs Asian studies, although only a few studies were evaluated.
As you can see from the table below, there is significant heterogeneity in terms of how PFS was presented to the cancer patient respondents.
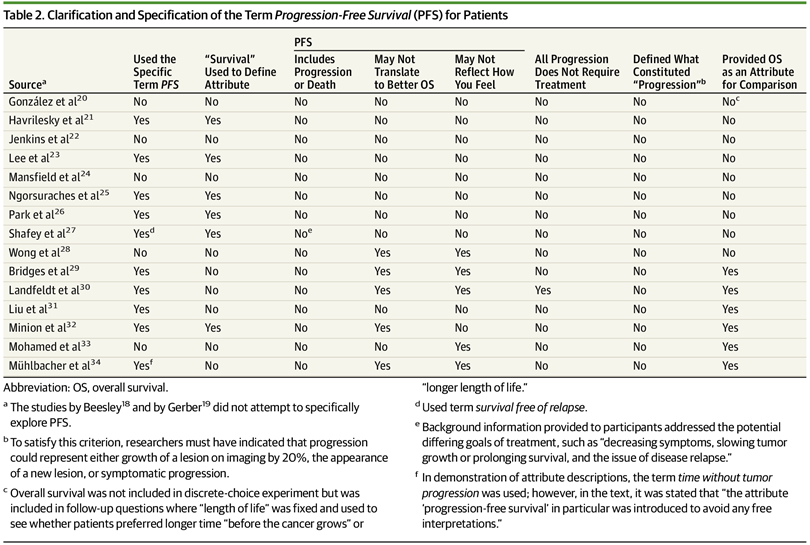
In some of the studies, PFS was the most important attribute. In others, quality of life factors were more important. Overall, however, it is clear that more research is needed to fully understand how patients perceive the value of PFS.

